Full Version PDF
Chief Complaint
Corneal ulcer in the left eye
History of Present Illness
A 10-year-old female referred to the ophthalmology clinic for worsening bacterial corneal ulcer while on fortified antibiotics for over one week and negative cultures.
Ocular History
- Corneal ulcer OS
- Exposure keratopathy OS s/p tarsorrhaphy
- Failed amniotic membrane graft
- Eye trauma OS
- Blepharitis OU
Past Medical History
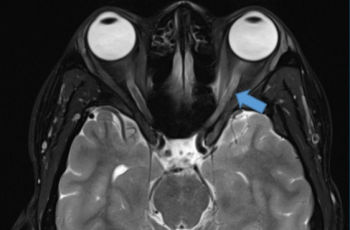
- Brain tumor s/p resection (several years ago)
Medications
- Artificial tears
- Moxifloxacin ophthalmic
- Fortified Tobramycin ophthalmic
- Fortified Vancomycin ophthalmic
Allergies
- No known drug allergies
Family History
- None
Social History
- None
Review of Systems
- As noted in HPI, otherwise negative
Ocular Exam
Physical Exam
|
|
OD | OS |
|---|---|---|
| Visual Acuity | 20/20 | HM |
| Intraocular Pressure (mmHg) | 13 | 11 |
| Pupils | Round, reactive | Round, reactive |
| Extraocular Motility | Full | Full |
Slit Lamp Exam
|
|
OD | OS |
|---|---|---|
| External | Normal | Lagopthalmos |
| Lids and Lashes | Normal, no lesions | Tr papillary reaction on upper/lower lid |
| Conjunctiva/Sclera | White and quiet | White and quiet |
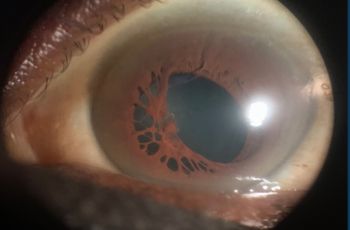
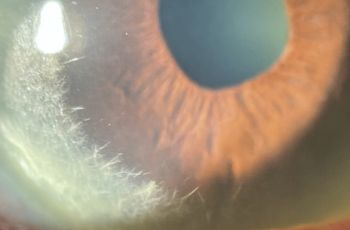
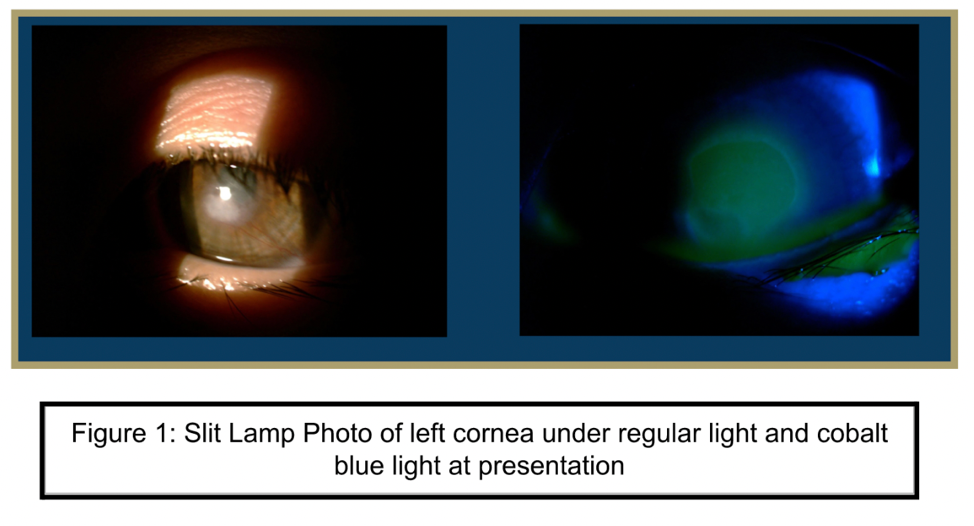
| Cornea | Clear | Epi defect 4.5 x 4mm, 6-7 o'clock dense white infiltrate with thinning |
| Anterior Chamber | Deep and quiet | Deep and quiet |
| Iris | Round and reactive | Round and reactive |
| Lens | Clear | Clear |
Imaging
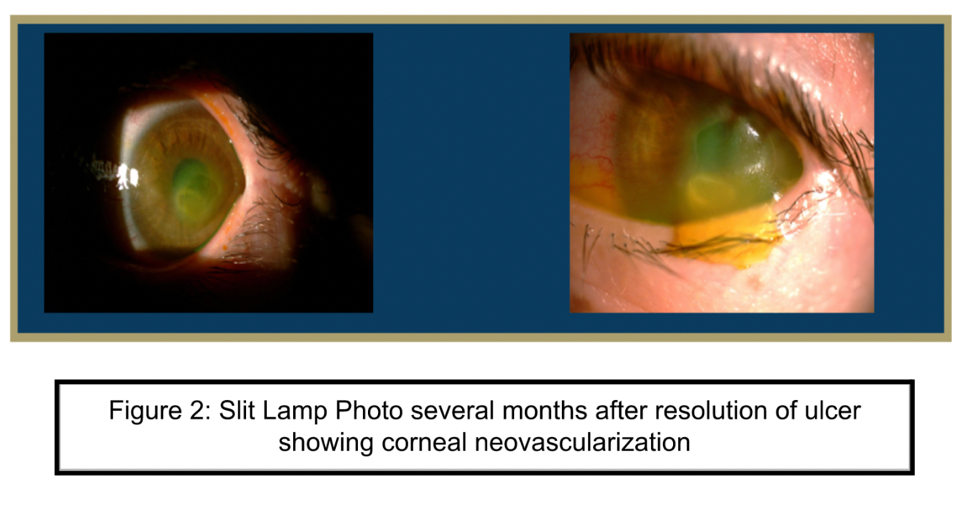
Laboratory Testing
- HSV 1/2 PCR: negative
- Aerobic Bacterial Culture: staphylococcus hominis in broth only
Differential Diagnosis
- Infectious corneal ulcer
- Neurotrophic corneal ulcer
Clinical Course
A 10-year-old female with a past medical history of a brain tumor with residual CN 5-7 palsy presented to the ophthalmology clinic as referral for corneal ulcer OS. She was initially treated with Vigamox but continued to have a worsening ulcer despite starting on topical fortified antibiotics and with negative cultures at one week. Her past ocular history was notable for a history of corneal ulcer OS, exposure keratopathy s/p tarsorrhaphy, and neurotrophic keratitis OS. She underwent a repeat infectious work up including an HSV1/2 PCR and aerobic bacterial culture, which returned positive only for staph hominis growth only in broth. After infection was ruled out, fortified antibiotics were tapered and she was started on low dose topical steroids and topical recombinant human nerve growth factor (cenegermin-bkbj ophthalmic solution). She was also advised to liberally lubricate the eye with drops and gel, take supplemental oral Vitamin C, and tape the eyelid at night throughout her course. The defect resolved and was complicated by corneal neovascularization which was managed and stabilized with topical steroids and cyclosporine. She continues to follow up on a regular basis for corneal monitoring and intraocular pressure checks.
Discussion
A corneal ulcer is a defect in the corneal epithelium and can be due to infectious or noninfectious causes. Among the infectious etiologies of ulcers, bacteria is the most common cause, with the most common pathogens being Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, and Pseudomonas aeruginosa (Byrd, 2022). Such pathogens typically enter through a break in the corneal epithelium, which can occur due to a number of mechanisms including contact lens wear and ocular trauma leading to corneal abrasions.
The two main differential diagnoses considered for this patient’s corneal ulcer were infectious and neurotrophic etiologies. Due to persistent symptoms following initial therapy for bacterial keratitis, the patient was started on cenegermin-bkbj ophthalmic solution, sold under the brand name of Oxervate. This drug contains recombinant human nerve growth factor and is the first-ever FDA approved topical medication for the treatment of neurotrophic keratitis (Deeks, 2020). Human nerve growth factor plays a significant role in the cornea by supporting corneal nerve cell growth and healing. Randomized control trials studying Oxervate have shown its efficacy in achieving complete corneal healing after just eight weeks of treatment, as was seen in the case of this patient.
As demonstrated by this patient’s clinical course, corneal ulcers may lead to a complication known as corneal neovascularization. Corneal neovascularization (cNV) is a sight-threatening condition characterized by the formation and subsequent stromal invasion of abnormal capillaries within the cornea. Corneal neovascularization affects 1.4 million people per year, 12% of whom suffer from vision loss. It can develop from traumatic, infectious, or degenerative damage to the distinctly avascular cornea. It can also present secondary to corneal ulcers, herpes infection, graft rejections, contact lenses, and surgery (Sharif, 2019). Normally, following epithelial injury, limbal stem cells are crucial for regenerating the corneal epithelium and maintaining corneal integrity through anti-angiogenic and immunomodulatory homeostatic mechanisms. However, in corneal neovascularization, apoptosis of limbal stem cells and inappropriate repair by these cells can occur, resulting in an excess production of inflammatory cytokines (IL-6, IL-7, IL-8) and vascular endothelial growth factor (VEGF) (Sharif, 2019). This imbalance between angiogenic and antiangiogenic factors leads to the presence of blood vessels in the cornea, which impair vision by blocking and diffracting light.
The incidence of the various etiologies underlying cNV vary in different parts of the world. In Western countries the most common etiology is herpes simplex keratitis, with approximately 500,000 cases of ocular herpes simplex virus reported every year (Lasagni, 2021). In regards to non infectious etiologies, contact lenses have been reported to be the most common non infectious cause of cNV in the US, affecting approximately 11–30% of the contact lens wearers (Lasagni, 2021). The patient presented in this case did not have any evidence of these common etiologies. The patient appears to be a demographic outlier as a recent study also showed that the median age of onset for cNV is 50.9 years of age (Lasagni, 2021).
Obtaining a diagnosis of cNV can be done in the clinical setting by slit lamp biomicroscopy under indirect and retro illumination. Neovascularization occurs rapidly, making early diagnosis difficult. Therefore, the risk for developing cNV should be assessed at regular time intervals during routine eye examinations. Among high-risk groups, such as contact lens wearers, screening should occur at shorter intervals to minimize the chance of vision loss (Sharif, 2019). In the present case, the patient received a routine slit lamp examination and intraocular pressure check every 6 months given her complicated ocular history of corneal ulceration and neurotrophic and infectious keratitis – all risk factors for cNV.
The cNV observed in this case must be understood in the context of the patient’s complex ocular history. Following surgical resection of an intracranial tumor, she developed CN 5-7 palsy. Consequently, trigeminal corneal innervation was damaged and severe corneal sensory impairment ensued, leading to a higher risk for corneal ulceration (Bonnie, 2003). With adequate corneal innervation, tear production and stimulation of the blinking reflex are crucial in maintaining an intact epithelium and minimizing external threats. However, in the absence of sufficient nerve function, the cornea is subject to insult from external stimuli, such as pathogens, xenobiotics, heat, and mechanical irritants (Yang, 2018). This explains the pathophysiological mechanism behind the patient’s presumed infectious keratitis. Certainly, corneal innervation is crucial for maintaining corneal health.
Finally, regarding the development of cNV in this patient, findings from several mouse model studies shed light on the possible mechanism at play. The cornea is avascular, yet remains the most densely innervated tissue in the body. Therefore, recent investigations have aimed to elucidate the relationship between nerves and neovessels. Interestingly, results demonstrate that both superficial and deep stromal corneal sensory nerves disappeared in those areas where neovessels were formed, while those in nonvascularized regions remained (Ferrari, 2013). Additionally, ablation of the ophthalmic branch of the trigeminal nerve led to cNV.
Injections and topical treatments can be used in the management of neovascularization. Steroids and anti-VEGF medications have been used as interventions for years. Topical steroids like dexamethasone have shown to be antiangiogenic and inhibit neovascularization in addition to their other anti-inflammatory effects. While steroids continue to be the mainstay of treatment, anti-VEGF therapies have begun to gain favor as a more specific therapy (Maddula, 2011). VEGF (vascular endothelial growth factor) is a growth factor that stimulates the formation of blood vessels. It has been implicated as a crucial factor in inflammatory neovascularization and anti-VEGF therapeutics have been developed in order to specifically target its proangiogenic effects (Maddula, 2011). Bevacizumab, a monoclonal antibody, is a commonly used anti-VEGF therapy. Previous studies on bevacizumab have shown a 36% reduction in neovascularization (Sharif, 2019).
Corneal transplantation is currently the most successful approach for treating cNV. However, the most common complication involved in this approach is the risk of transplant rejection. This risk is exacerbated by the presence of cNV, making this patient population a high-risk transplant recipient. It has been observed that the extent of neovascularization directly correlates to the risk of rejection. In a study dividing patients into mild, moderate and heavy neovascularization, 65% of the transplants were rejected in the heavy neovascularization group, despite immunosuppressive therapy (Di Zazzo, 2016).
In addition to transplant, another treatment option for neovascularization includes laser phototherapy. An argon laser can be used to pass through the cornea with the hemoglobin from new vessels absorbing the argon energy. The absorption of this energy causes vessels to coagulate, reversing the neovascularization (Sharif, 2019). Photodynamic therapy is a treatment that combines a photosensitizing compound, light, and oxygen. The compound is absorbed by the neovascular tissue and is activated through an additional laser treatment, causing free radicals to be released, which destroy the surrounding neovascular tissue and reverse the cNV (Sharif, 2019). Though laser phototherapy options have been used frequently in the treatment of neovascularization, more studies need to be conducted in order to confirm its efficacy in comparison to other approaches to neovascularization treatment.
Overall, cNV is a sight-threatening condition with several primary and secondary etiologies, making early diagnosis challenging. High risk patients, such as those with herpes simplex keratitis, corneal ulcers, or contacts should be routinely examined for cNV via slit lamp examination and imaging. As for the young patient presented here, cNV and the preceding neurotrophic and infectious keratitis were likely a result of impaired corneal innervation due to trigeminal nerve palsy. This case is a reminder of the importance of corneal innervation in preserving and maintaining corneal health.
References
Bonini, S., Rama, P., Olzi, D., & Lambiase, A. (2003). Neurotrophic keratitis. Eye, 17(8), 989–995. https://doi.org/10.1038/sj.eye.6700616
Byrd LB, Martin N. Corneal Ulcer. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan.
Deeks ED, Lamb YN. Cenegermin: A Review in Neurotrophic Keratitis. Drugs. 2020 Apr;80(5):489-494. doi: 10.1007/s40265-020-01289-w. PMID: 32185680.
Di Zazzo, A., Kheirkhah, A., Abud, T. B., Goyal, S., & Dana, R. (2017). Management of high-risk corneal transplantation. Survey of ophthalmology, 62(6), 816–827. https://doi.org/10.1016/j.survophthal.2016.12.010
Ferrari, G., Hajrasouliha, A. R., Sadrai, Z., Ueno, H., Chauhan, S. K., & Dana, R. (2013). Nerves and neovessels inhibit each other in the cornea. Investigative Ophthalmology & Visual Science, 54(1), 813. https://doi.org/10.1167/iovs.11-8379
Khodadoust, A. A., & Silverstein, A. M. (1972). Studies on the nature of the privilege enjoyed by corneal allografts. Investigative ophthalmology, 11(3), 137–148.
Lasagni Vitar, R. M., Triolo, G., Fonteyne, P., Acuti Martellucci, C., Manzoli, L., Rama, P., & Ferrari, G. (2021). Epidemiology of Corneal Neovascularization and Its Impact on Visual Acuity and Sensitivity: A 14-Year Retrospective Study. Frontiers in medicine, 8, 733538. https://doi.org/10.3389/fmed.2021.733538
Maddula, S., Davis, D. K., Maddula, S., Burrow, M. K., & Ambati, B. K. (2011). Horizons in therapy for corneal angiogenesis. Ophthalmology, 118(3), 591–599. https://doi.org/10.1016/j.ophtha.2011.01.041
Sharif, Z., & Sharif, W. (2019). Corneal neovascularization: updates on pathophysiology, investigations & management. Romanian journal of ophthalmology, 63(1), 15–22.
Yang, A. Y., Chow, J., & Liu, J. (2018). Corneal Innervation and Sensation: The Eye and Beyond. The Yale journal of biology and medicine, 91(1), 13–21.
Chief Complaint
Second opinion for choroiditis OD and new findings OS
History of Present Illness
A 37-year-old female was referred to the ophthalmology clinic for “uveitis.” She complained of photophobia, floaters, and deep eye pain in the left eye. She denied any flashes or peripheral vision loss.
Ocular History
- CE/IOL OD (2019)
- Uveitis History:
- 2017: First episode of uveitis OD while pregnant. Followed locally and received a uveitis work-up which was negative. Treated topically and with local steroid injection (STK).
- 2019: flare OD. Treated topically and with PO prednisone.
- 2022: first flare OS. Moved to DC and referred to GW.
Past Medical History
- Hypothyroidism
Medications
- Levothyroxine
- Prednisone 40 mg
Allergies
- None
Family History
- None
Social History
- Born and raised in India
- Denies tobacco, alcohol or drug use
Review of Systems
- Denies SOB, cough, rash, joint pain/swelling, fevers, chills, weight loss
Ocular Exam
Physical Exam
|
|
OD | OS |
|---|---|---|
| Visual Acuity | 20/30+, PHNI | 20/80, PH 20/60- |
| Intraocular Pressure (mmHg) | 16 | 14 |
| Pupils | PERRL no APD | PERRL no APD |
| Extraocular Motility | Full | Full |
| Confrontational Visual Fields | Full | Full |
Slit Lamp Exam
|
|
OD | OS |
|---|---|---|
| External | Normal | Normal |
| Lids and Lashes | Normal, no lesions | Normal, no lesions |
| Conjunctiva/Sclera | White and quiet | White and quiet |
| Cornea | Clear | Clear |
| Anterior Chamber | Deep and quiet | Deep, trace cell/flare |
| Iris | Round and reactive | Round and reactive |
| Lens | PC/IOL | Clear |
Dilated Fundus Exam
|
|
OD | OS |
|---|---|---|
| Cup to Disc | 0.3 | 0.3 |
| Optic Nerve | Pink/sharp | Pink/sharp |
| Vessels | Attenuation | Attenuation |
| Macula | Punched out atrophic pigmented lesions | |
| Periphery | Multiple punched out scattered atrophic pigmented lesions | Multiple punched out white circular lesions with associated retinal hemorrhages with active chorioretinitis appearance |
| Vitreous | Tr haze | 1-2+ haze |
Imaging
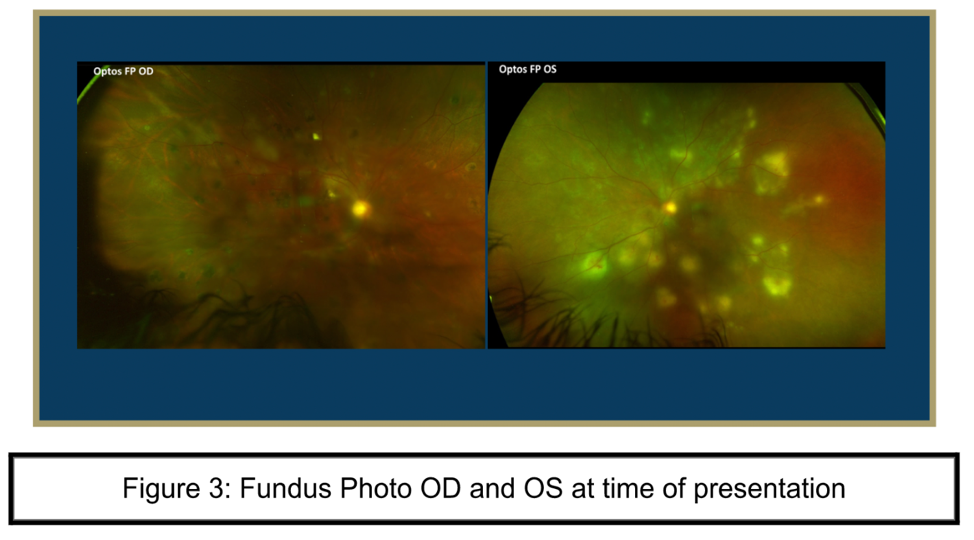
Fundus photo OD: has a slightly hazy media, nerve margins appear sharp but difficult to appreciate due to haze, slight attenuation of vessels, and multiple punched out atrophic pigmented lesions scattered in macula and periphery
Fundus photo OS: with hazy media, pink nerve perfused with sharp margins, attenuation of vessels, and multiple whitish circular lesions in periphery and midperiphery associated with retinal hemorrhages. These areas were concerning for active chorioretinitis.
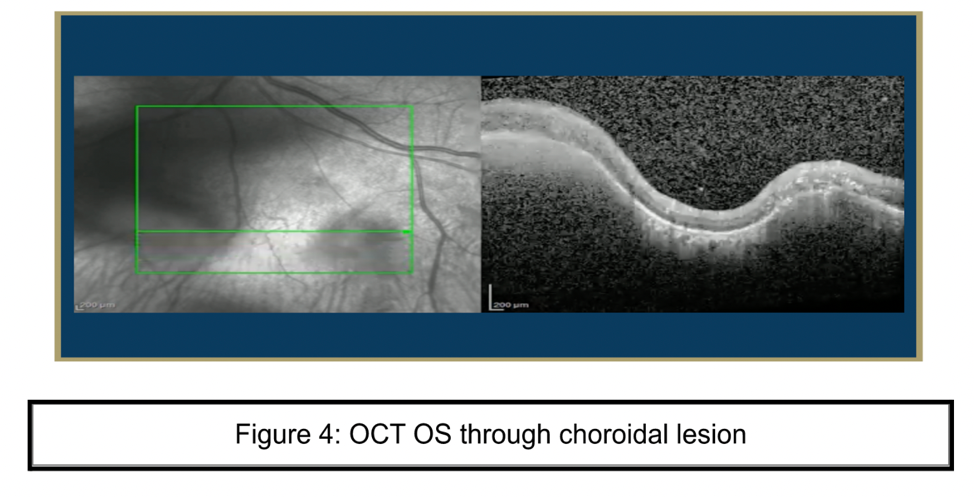
Laboratory Testing
- Tuberculosis
- Histoplasmosis
- Toxocariasis
- Sarcoidosis
- Syphilis
- Toxoplasmosis
- Serpiginous choroidopathy
- Acute retinal necrosis.
Differential Diagnosis
- Branch Retinal Artery Occlusion (BRAO)
- Transient monocular visual loss due to retinal artery vasospasm
- Hypertensive retinopathy OU
Clinical Course
A 37 year-old female with a previous history of two episodes of uveitis in the right eye was referred to the ophthalmology clinic for a second opinion regarding new findings in the left eye. Previous work up of uveitis had been negative. Social history was significant for being born and raised in India. Her constellation of history and exam findings of choroidal lesions were most concerning for the diagnosis of TB serpiginous-like choroiditis vs serpiginous choroiditis. An initial TB test years prior was negative, but was repeated and returned positive. She was referred to the infectious disease clinic for initiation of appropriate anti-tubercular treatment (ATT).
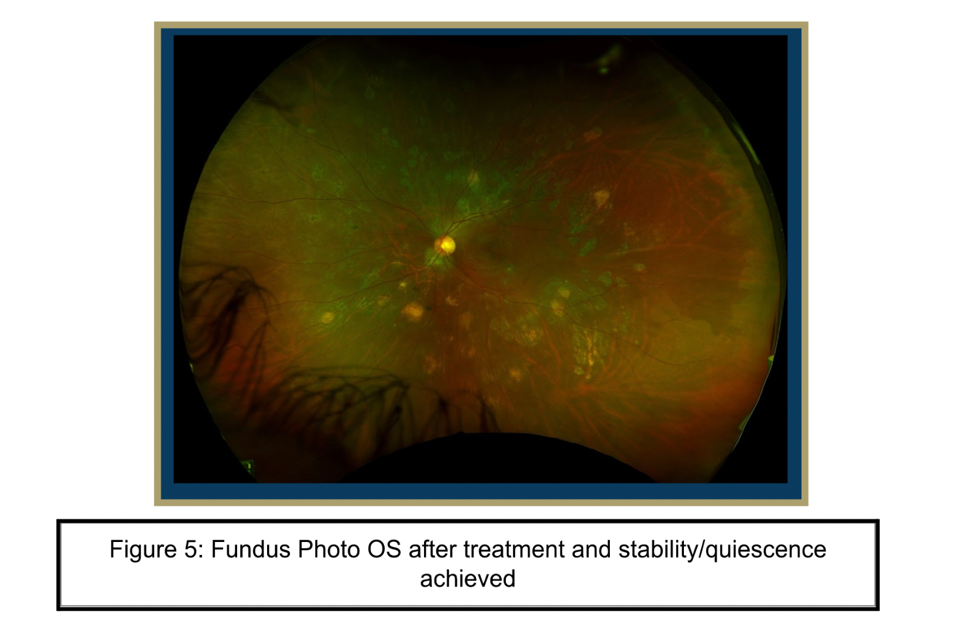
In addition to her course of ATT, her anti-inflammatory therapy was augmented with an increase in the oral prednisone dose to 50 mg daily. She was also started on prednisolone acetate four times a day in the left eye and received a subtenon kenalog injection in the left eye. She achieved quiescence with improvement in visual acuity and symptoms. Due to an elevation in her Liver Function Tests (LFTs), the patient took a two week break from ATT. She returned to the ophthalmology clinic weeks later with a flare in the left eye involving the macula, which was successfully managed with an intravitreal triamcinolone injection and topical dorzolamide-timolol in the left eye. On the most recent follow up, the patient was continuing ATT. The lesions on OCT and examination were quiet. She was continued on dorzolamide-timolol BID in the left eye and the oral prednisone dose was decreased to 7.5 mg daily.
Discussion
Serpiginous like choroiditis (SLC) is an inflammation of the choroid that leads to loss of choriocapillaris, atrophy of overlying retinal pigment epithelium (RPE), and degeneration of photoreceptor cells with adhesion of the degenerated retina to the choroid. These lesions of atrophy vary in size and location, often presenting asymmetrically and bilaterally. Newer developing and older healing lesions may be present simultaneously. Recurrences of choroidal inflammation can present over months and years, with absent intervention leading to foveal involvement and loss of vision. There are several possible etiologies including autoimmune, bacterial, or viral origins. Mycobacterium infection is a known precipitant of SLC and may be related to the bacilli being sequestered in the RPE. However, isolation of the bacteria from eyes remains difficult, making definitive diagnosis by culture rare. Autoreactive T cells have also been identified in the pathogenesis of TB-associated uveitis and may play a similar role in TBSLC. The pathogenesis of TBSLC remains unclear. However, the choroiditis associated with active and latent systemic TB infection may represent a focal reactivation of the mycobacterium.
The classic presentation of SLC is a young to middle aged man from an endemic TB country with a previous history of active or latent TB infection. The patient typically presents with a chief complaint of diminished central vision, metamorphopsia or a scotoma, in contrast to the patient in this case, whose central and peripheral vision were preserved. Many patients present asymptomatically until lesions start involving the macula. In this case, the patient’s recurring flares demonstrate another presentation of TBSLC as choroidal inflammation waxes and wanes over time.
On ophthalmic examination, active SLC lesions on the retina are described as “yellowish white in color” with elevated borders, while the “center of the lesion is relatively flat, with pigmentary changes at the level of the RPE suggestive of a healing process in the center'' (Agarwal 2020). The lesions are initially noncontiguous and show a “wave-like progression over a period of 1-4 weeks,” eventually becoming confluent.
Confirmatory studies for SLC focus on identifying an active TB infection that is the source of the ophthalmological symptoms. These can be done through tuberculin skin (TST) or blood tests. Patients may present with positive TST with normal chest radiographs, and no evidence of tuberculosis elsewhere. CT scans may increase the likelihood of detecting pulmonary tuberculosis while PET scans may be more sensitive at detecting hilar adenopathy when establishing TB diagnosis. In order to determine the extent of ocular damage, optical coherence tomography (OCT), fundus autofluorescence (FAF), and OCT angiography are useful. Fundus appearance is diagnostic of the SLC. Definitive diagnosis of TBSLC is done by culture of mycobacterium isolated from the diseased eye. Presumptive TB diagnosis can include a positive skin test or radiographic evidence of TB lung involvement. In this patient, TB test results were inconclusive and other causes of uveitis were ruled out, thus requiring clinicians to deduce the definitive diagnosis from the patient’s history and response to treatment.
Anti-tubercular therapy (ATT) is indicated in TBSLC treatment and reduces future recurrences of inflammation. However, initiation of ATT can result in paradoxical worsening of choroiditis due to inflammatory reaction. As such, immunosuppressive therapy should be coadministered, either systemically with oral corticosteroid therapy, or as more recently demonstrated, locally via intravitreal dexamethasone implant. In this case, intravitreal triamcinolone injection and an increased dosage of prednisone were effective in controlling inflammation. Several aspects of the disease course are variable, including duration of infection, time to treatment, treatment response, and the specific regions of the retina that are affected.
Serpiginous like choroiditis is a rare disease with much of its pathophysiology, risk factors and best available treatment practices still being understood. There are many diseases that are more common and can present similarly to SLC, causing this rare disease to be overlooked. Our patient had to see many ophthalmologists before a diagnosis could be made. High clinical suspicion and repeat testing was critical in solving this case.
References
Agarwal, Aniruddha MD*; Aggarwal, Kanika MS*; Mandadi, Spoorti Krishna Reddy MBBS*; Kumar, Aman MBBS*; Grewal, Dilraj MD†; Invernizzi, Alessandro MD‡,§; Bansal, Reema MS*; Sharma, Aman MD; Sharma, Kusum MD**; Gupta, Vishali MS*; for OCTA Study Group. Longitudinal Follow-Up of Tubercular Serpiginous-like Choroiditis using Optical Coherence Tomography Angiography. Retina 41(4):p 793-803, April 2021. doi: 10.1097/IAE.0000000000002915
Agarwal A, Marchese A, Rabiolo A, Agrawal R, Bansal R, Gupta V. Clinical and Imaging Factors Associated With the Outcomes of Tubercular Serpiginous-like Choroiditis. Am J Ophthalmol. 2020 Dec;220:160-169. doi: 10.1016/j.ajo.2020.07.024. Epub 2020 Jul 23. PMID: 32710829.
Arrieta-Bechara C, Haro-Álvarez B, Cocho-Archiles L, Herreras Cantalapiedra JM. Clinical case: Serpiginous-like choroiditis with macular involvement and good response after treatment with adalimumab. Arch Soc Esp Oftalmol (Engl Ed). 2022 Aug;97(8):477-480. doi: 10.1016/j.oftale.2021.02.011. Epub 2022 Feb 10. PMID: 35914892.
Bansal, R., Gupta, V. Tubercular serpiginous choroiditis. J Ophthal Inflamm Infect 12, 37 (2022). https://doi.org/10.1186/s12348-022-00312-3
Brar M, Sharma M, Grewal S, Grewal D, Dogra MR. Longitudinal study of serpiginous choroiditis and serpiginous like choroiditis using wide field OCT angiography. European Journal of Ophthalmology. 2022;32(3):1555-1561. doi:10.1177/11206721211028529
Dutta Majumder, P., Biswas, J., & Gupta, A. (2019). Enigma of serpiginous choroiditis. Indian journal of ophthalmology, 67(3), 325–333. https://doi.org/10.4103/ijo.IJO_822_18
Kawali A, Emerson GG, Naik NK, Sharma K, Mahendradas P, Rao NA. Clinicopathologic Features of Tuberculous Serpiginous-like Choroiditis. JAMA Ophthalmol. 2018;136(2):219–221. doi:10.1001/jamaophthalmol.2017.5791
Mishra, S., & Biswas, J. (2020). Peripheral serpiginous like choroiditis: A unique and unheard entity. Indian journal of ophthalmology, 68(5), 911–912. https://doi.org/10.4103/ijo.IJO_1518_19
Nazari Khanamiri, H., & Rao, N. A. (2013). Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Survey of ophthalmology, 58(3), 203–232. https://doi.org/10.1016/j.survophthal.2012.08.008
Vianna, R.N.G., Vanzan, V., da Fonsêca, M.L.G. et al. Unilateral macular serpiginous-like choroiditis as the initial manifestation of presumed ocular tuberculosis. Int J Retin Vitr 7, 1 (2021). https://doi.org/10.1186/s40942-020-00272-7
Acknowledgments
Case Report Authors
Case 1: Corneal Neovascularization
Yusuf Bade, BA MS1
Meredith Wells, BS MS2
Case 2: Tuberculosis Uveitis
Sasha Narain, BA MS2
Jacob Diaz, BS MS2
Resident Editors
Masumi Asahi, DO
Braedon Murdock, MD
Will Foos, MD
Attending Reviewer
David Belyea, MD MBA
Editors in Chief
Jason Dossantos, MS3
Julie Thomasian, MS4