Full Version PDF
Chief Complaint
Blurry vision in the left eye
History of Present Illness
A 50-year-old female with a history of multiple sclerosis (MS), type 2 diabetes mellitus, antiphospholipid antibody syndrome, and chronic renal failure presents to the ophthalmology clinic with a 6-day history of left eye blurry vision.
Ocular History
- Bilateral proliferative diabetic retinopathy
Past Medical History
- Optic neuritis (2 episodes in last 27 years)
- Multiple Sclerosis
Medications
- Siponimod
Allergies
- No known drug allergies
Family History
- None
Social History
- None
Review of Systems
- As noted in HPI, otherwise negative
Ocular Exam
Physical Exam
|
|
OD | OS |
|---|---|---|
| Visual Acuity | Count Fingers | 20/60 |
| Intraocular Pressure (mmHg) | 16 | 16 |
| Pupils | PERRL, no APD | PERRL, no APD |
| Extraocular Motility | Full | Full |
Dilated Fundus Exam
|
|
OD | OS |
|---|---|---|
| Cup to Disc | 0.3 | 0.3 |
| Optic Nerve | Pallor | Pallor |
| Vessels | Normal caliber and course | Normal caliber and course |
| Macula | Flat, media clear, no other inflammatory or retinal findings | Flat, media clear, no other inflammatory or retinal findings |
| Periphery | 360 degrees of pan-retinal photocoagulation scars | 360 degrees of pan-retinal photocoagulation scars |
| Vitreous | Clear | Clear |
Imaging
Obtained from the following BMJ article:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9660509
Differential Diagnosis
- Diabetic macular edema
- Macula edema associated with MS modifying drugs
- Breakthrough optic neuritis
Clinical Course
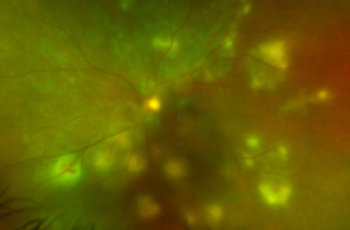
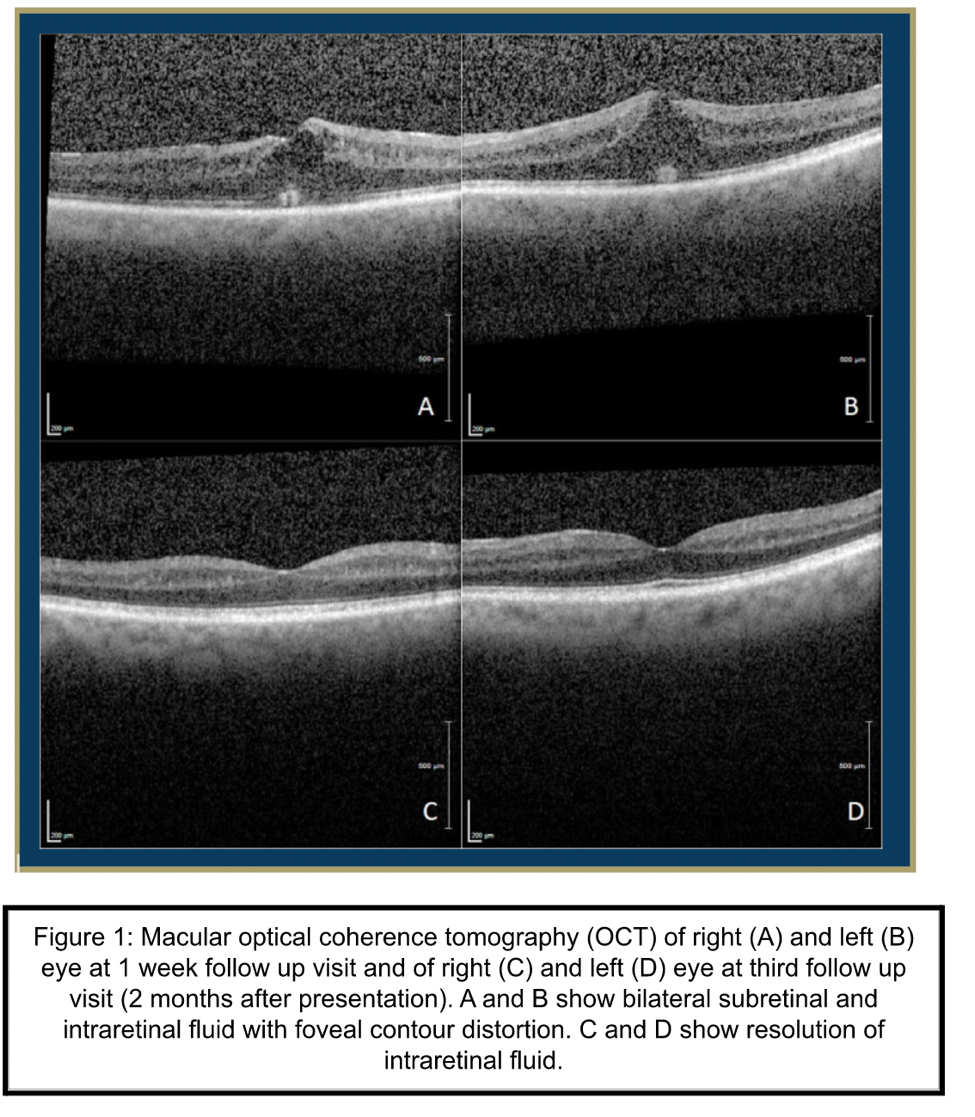
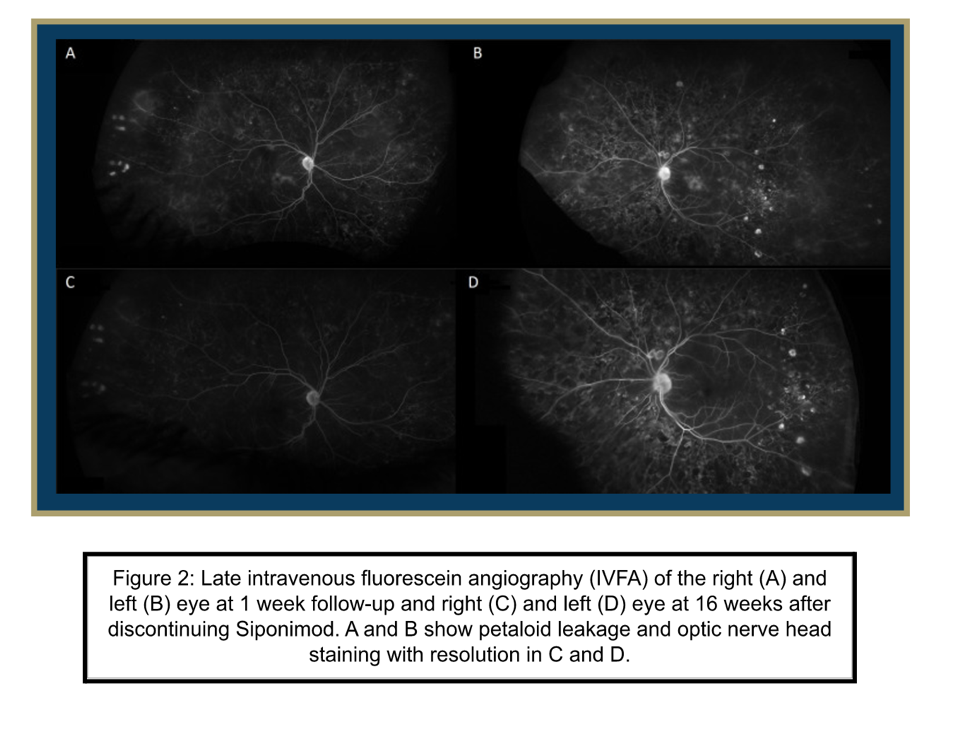
A 50-year-old female with a history of multiple sclerosis (MS) presented to the ophthalmology clinic with blurry vision OS. Clinical exam, including OCT imaging and IVFA, revealed cystoid macular edema with concerns for drug-related cystoid macular edema or breakthrough optic neuritis. Her past medical history was notable for beginning siponimod for relapse-remitting MS five months prior to current presentation. She was treated with topical ketorolac 0.5% and prednisolone acetate 1% twice daily in her left eye with follow-up to a retina specialist after one week. The patient’s eye drops were then increased to four times daily in both eyes. Due to concern for drug-related cystoid macular edema, neurology was consulted to discuss changing siponimod to another disease modifying agent. Neurology discontinued siponimod and started dimethyl fumarate. Three weeks after her initial presentation and discontinuation of siponimod, the patient demonstrated interval resolution of edema on OCT imaging and noted subjective improvement of her vision despite unchanged best-corrected visual acuity (BCVA).
Her eye drops were tapered over a course of three weeks, and at two-months follow-up her macular edema was completely resolved with improvement of left eye vision. She continued on dimethyl fumarate by her neurologist for management of her MS and had no subsequent recurrence of cystoid macular edema.
Discussion
Cystoid macular edema (CME) is a form of macular retinal thickening characterized by cystic accumulation of fluid in the intraretinal space due to a disturbance of the blood retinal barrier (Hollo, 2020). Although the exact cause of CME is not known, it is often found alongside risk factors that disrupt the normal interactions of the delicate retinal environment, such as diabetes, retinal vein occlusion, uveitis, and certain disease-modifying therapies – as seen in the present case. Existing literature has documented cases of drug-associated CME in patients using trastuzumab, paclitaxel, fingolimod and siponimod. These patients can present with either unilateral or bilateral blurred vision and decreased visual acuity, or without symptoms altogether. Drug-associated CME is usually diagnosed with a dilated retinal exam, optical coherence tomography (OCT) or Intravenous fluorescein angiography (FA) showing subretinal fluid, optic disc staining, dye leakage/petaloid macular leakage, intraretinal fluid, or foveal cysts. In the setting of comorbid ophthalmic inflammatory diseases and diabetic retinopathy, drug-associated CME can be hard to diagnose given similar presentations on clinical exam. Standard treatment for drug-associated CME involves drug cessation, with variation of time to resolution, although other additional treatment options have been documented such as topical non-steroidal anti-inflammatory drugs and steroids (Afshar, 2013).
To date, drug-associated CME has been commonly associated with fingolimod, a disease-modifying drug for relapse-remitting MS whose active metabolites binds to four sphingosine-1-phosphate receptor subtypes on lymphocytes, thereby preventing degradation of central nervous system myelin (Munoz-Ortiz, 2021; Wang, 2022). The time to presentation of fingolimod-associated macular edema (FAME) from drug initiation can vary greatly – usually occurring three months after initiation, but patients can have late onset CME presenting one year after drug initiation (Wang, 2022). Additionally, one case report documented a 42 year-old patient with MS who demonstrated symptoms of FAME as early as 24 hours post- fingolimod initiation (Soliman, 2016). Currently, the Food and Drug Administration (FDA) recommends patients have an ophthalmological examination before initiating fingolimod and at three to four months after initiation (Jain, 2012). If clinical suspicion for FAME is high, the patient should return for periodic eye exams, however the optimal timeline for follow-up has yet to be established (Wang, 2022). Patients who have a history of diabetes mellitus, uveitis, or are on another therapy associated with CME would most likely benefit from closer monitoring, as they are at a higher risk for developing FAME (Gaskin, 2015; Wang, 2022).
Siponimod is a newer disease modifying agent for MS that selectively binds two sphingosine-1-phosphate receptor subtypes and has an improved side effect profile from its predecessor, fingolimod. Despite the improved adverse effects, the drug’s similar mechanism of action prompts concerns for macular edema associations. Previous literature shows a documented case of a 54 year-old female with MS who developed macular edema three months (Rettler, 2021) after starting siponimod therapy. Furthermore, during the phase 2 dose-blinded, randomized extension of the BOLD study, there was one case of macular edema documented in the setting of siponimod usage. This patient had a history of uveitis and was on 10 mg of siponimod therapy – the highest dose of any participant in the trial. (Kappos, 2016). In the subsequent EXPAND trial, a phase 3 double-blind, randomized study, investigators reported an increased incidence of macular edema in siponimod-treated MS patients when compared to the placebo group (2% vs <1%) (Cao, 2021). Consequently, the FDA recommends an ophthalmic evaluation prior to receiving the first dose of siponimod and reevaluation if there are any changes in vision during the course of treatment (2019).
This case importantly underscores how a history of diabetic retinopathy, ocular inflammatory disorders, and microvascular changes can complicate and obscure a timely diagnosis of CME. In the setting of this patient’s diabetic retinopathy, an already inflamed and compromised blood retinal barrier likely contributed to her delay in onset at five months after starting siponimod, as compared to the three to four month time-to-diagnosis found in the BOLD and EXPAND trials.
To conclude, here we highlight one of the first documented cases of bilateral siponimod-associated macular edema in a setting of stable diabetic retinopathy and multiple sclerosis which was successfully treated with drug cessation and topical anti-inflammatory agents. This case serves as an important reminder for the careful management and close examination of patients on siponimod with concomitant diabetes or uveitis.
References
Original Case Report published by GW Department of Ophthalmology:
Foos, W. F., Culp, C., Asahi, M., & Patronas, M. (2022). Siponimod-related bilateral cystoid macular oedema and intravenous fluorescein angiographic findings in a patient with stable proliferative diabetic retinopathy without history of diabetic macular oedema. BMJ case reports, 15(11), e251066. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9660509/).
-------------------------------------------------------------------------------------------------------------------------------
Afshar AR, Fernandes JK, Patel RD, Ksiazek SM, Sheth VS, Reder AT, Hariprasad SM. Cystoid macular edema associated with fingolimod use for multiple sclerosis. JAMA Ophthalmol. 2013 Jan;131(1):103-7. doi: 10.1001/jamaophthalmol.2013.570. PMID: 23307220.
Cao L, Li M, Yao L, Yan P, Wang X, Yang Z, Lao Y, Li H, Yang K, Li K. Siponimod for multiple sclerosis. Cochrane Database of Systematic Reviews 2021, Issue 11. Art. No.: CD013647. DOI: 10.1002/14651858.CD013647.pub2. Accessed 09 March 2023.
Gaskin JC, Coote M. Postoperative cystoid macular oedema in a patient on fingolimod. BMJ Case Rep. 2015 May 12;2015:bcr2015210415. doi: 10.1136/bcr-2015-210415. PMID: 25969500; PMCID: PMC4434361.
Holló G, Aung T, Cantor LB, Aihara M. Cystoid macular edema related to cataract surgery and topical prostaglandin analogs: Mechanism, diagnosis, and management. Surv Ophthalmol. 2020;65(5):496-512. doi:10.1016/j.survophthal.2020.02.004
Jain N, Bhatti MT, Tariq Bhatti M. Fingolimod-associated macular edema: incidence, detection, and management. Neurology 2012;78:672–80. 10.1212/WNL.0b013e318248deea
Kappos L, Li DK, Stüve O, Hartung HP, Freedman MS, Hemmer B, Rieckmann P, Montalban X, Ziemssen T, Hunter B, Arnould S, Wallström E, Selmaj K. Safety and Efficacy of Siponimod (BAF312) in Patients With Relapsing-Remitting Multiple Sclerosis: Dose-Blinded, Randomized Extension of the Phase 2 BOLD Study. JAMA Neurol. 2016 Sep 1;73(9):1089-98. doi: 10.1001/jamaneurol.2016.1451. PMID: 27380540.
Kim MK, Kim US. Analysis of fundus photography and fluorescein angiography in nonarteritic anterior ischemic optic neuropathy and optic neuritis. Korean J Ophthalmol 2016;30:289–94
Muñoz-Ortiz J, Reyes-Guanes J, Zapata-Bravo E, et al. Ocular adverse events from pharmacological treatment in patients with multiple sclerosis-A systematic review of the literature. Syst Rev. 2021;10(1):280. Published 2021 Oct 28. doi:10.1186/s13643-021-01782-7
Rettler, M., & Gratton, S.Siponimod associated macular edema in a patient with multiple sclerosis. Neuroimmunology Reports. 2021.
Soliman MK, Sarwar S, Sadiq MA, Jack L, Jouvenat N, Zabad RK, Kedar S, Nguyen QD. Acute onset of fingolimod-associated macular edema. Am J Ophthalmol Case Rep. 2016 Sep 28;4:67-70. doi: 10.1016/j.ajoc.2016.09.005. PMID: 29503930; PMCID: PMC5757484.
U.S. Food and Drug Administration (2019). Label: Mayzent (siponimd) tablets, for oral use. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209884s000lbl.pdf.
Wang C, Deng Z, Song L, Sun W, Zhao S. Diagnosis and Management of Fingolimod-Associated Macular Edema. Front Neurol. 2022 Jul 15;13:918086. doi: 10.3389/fneur.2022.918086. PMID: 35911881; PMCID: PMC9334868
Chief Complaint
Crystalline Keratopathy
History of Present Illness
A 74-year-old female with a history of normal-tension glaucoma (NTG) and uveitis presents to the ophthalmology clinic for follow-up after being lost to follow-up for several years. She denies any significant changes in vision, flashing lights, floaters, eye pain, or photophobia.
Ocular History
- Normal-Tension Glaucoma OU
- Uveitis OU
- Dry eye syndrome (DES)/Meibomian gland dysfunction (MGD) OU
- Cataracts (OU)
Past Medical History
- Hypertension
- Hyperlipidemia
- Migraine
Medications
- Brimonidine 0.1% ophthalmic solution
- Carboxymethylcellulose 1% ophthalmic solution
- Prednisolone acetate 1% ophthalmic suspension
- Travoprost 0.004% ophthalmic solution
- Aspirin 81 mg QD
- Atorvastatin 10 mg
- Hydrochlorothiazide 12.5 mg QD
- Rizatriptan 5 mg
Allergies
- None
Family History
- Glaucoma
Social History
- None
Review of Systems
- Negative
Ocular Exam
Slit Lamp Exam
|
|
OD | OS |
|---|---|---|
| External | Normal | Normal |
| Lids and Lashes | Normal, no lesions | Normal, no lesions |
| Conjunctiva/Sclera | White and quiet | White and quiet |
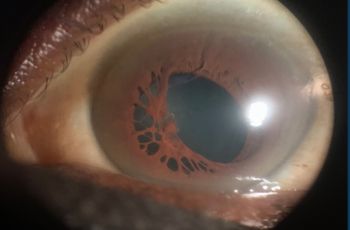
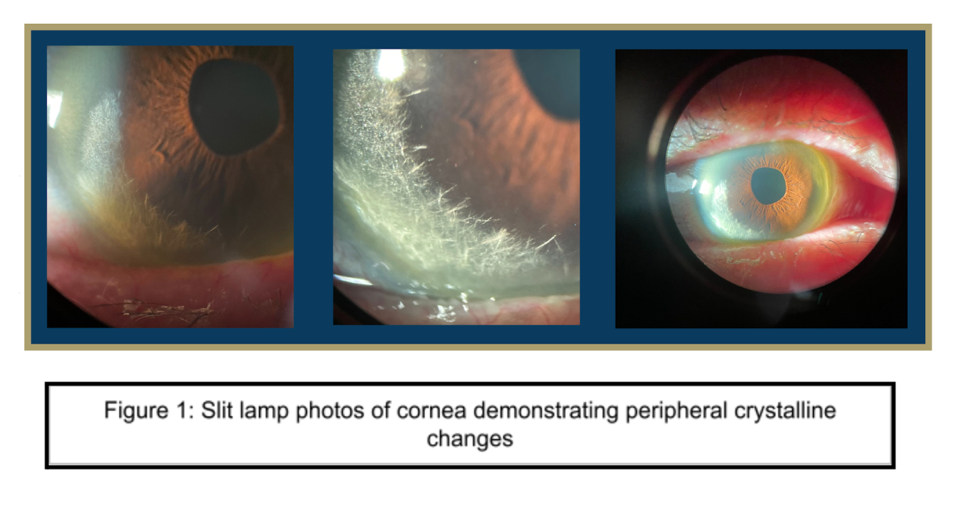
| Cornea | Crystalline changes peripheral | Crystalline changes peripheral |
| Anterior Chamber | Deep and quiet | Deep and quiet |
| Iris | Round and reactive | Round and reactive |
| Lens | 3+ Nuclear Sclerosis | 3+ Nuclear Sclerosis |
Imaging
Differential Diagnosis
- Lattice Corneal Dystrophy / Amyloidosis
- Infectious Crystalline Keratopathy
- Schnyder corneal dystrophy
Clinical Course
A 74-year-old female with a history of normal tension glaucoma (NTG), uveitis, and cataracts presented to glaucoma clinic for an appointment after being lost to follow-up for several years. The patient had been compliant with medications for NTG, including Travatan Z and Brimonidine BID, and the intraocular pressure remained stable. Slit lamp examination of the cornea revealed bilateral peripheral crystalline changes associated with marginal degeneration and pannus. The patient was referred to cornea clinic for further evaluation. Several possible etiologies of the crystalline keratopathy were considered, including Schnyder corneal dystrophy in the context of an elevated lipid panel and a plasma cell dyscrasia due to a recently elevated free kappa chain/lambda ratio and elevated protein on urine electrophoresis. Notably, the patient reported no known family history of corneal dystrophies or systemic metabolic disorders. Given the patient’s longstanding history of topical steroid use for granulomatous uveitis, work-up for infectious crystalline keratopathy was also considered. However, the patient was subsequently lost to follow-up.
Discussion
In the present case study, the patient was found to have crystalline keratopathy, which was associated with marginal degeneration and pannus. Although the differential diagnosis was broad, infectious crystalline keratopathy was explored given the patient’s complex ocular history of granulomatous uveitis and the associated long-term use of topical steroids. Infectious crystalline keratopathy (ICK) is a corneal infection characterized by the formation of branching, needle-like opacities within the corneal stroma, typically associated with infections caused by slow-growing, low-virulence organisms (Porter, 2018). The most common causative agent is alpha-hemolytic Streptococcus viridans. Other causes include additional alpha-hemolytic Streptococcus species, coagulase-negative Staphylococci, and other gram-positive bacteria. Fungal and protozoal organisms can also cause ICK, albeit less frequently (Bowling, 2016).
Patients with ICK often experience loss of vision, photophobia, and edema, along with pain that can range from minor discomfort to severe. This condition can affect individuals of any age but is more prevalent in those with a history of contact lens wear, corneal surgery, or an immunocompromised state (Kinota, 1993). In particular, ICK is most frequently observed after penetrating keratoplasty, and its association with long-term use of localized glucocorticoids has been noted. Any predisposing factors can compromise the corneal epithelium, allowing pathogens to invade the corneal stroma and establish an infection (Weisenthal, 2016)
Physical examination of the eye in ICK may show chemosis, an inflamed conjunctiva, and unique needle-like, branching deposits in the frontal part of the cornea. The diagnosis of ICK is based on clinical findings supported by growing cultures of the causative organism from corneal scrapings or diagnostic keratectomy. One notable characteristic that distinguishes ICK from other types of chronic infectious bacterial keratitis is the presence of biofilm as these are rarely seen in other forms of keratitis. In ICK, transmission electron microscopy with specialized fixatives is used to reveal the presence of biofilm. The biofilm, which is rich in polysaccharides, stains strongly with periodic acid-Schiff stain, while showing a weak reaction to gram stain (Gorovoy, 1983). Additionally, in vivo confocal microscopy has emerged as a useful diagnostic tool for all types of keratitis and can aid in identifying the presence of needle-like crystalline opacities in ICK. This non-invasive imaging technique allows for real-time visualization of the corneal layers and can not only detect the presence of crystalline deposits but also detect inflammatory cells (Villani, 2014).
Treatment of ICK requires targeted antimicrobial therapy based on cultures and sensitivity test results. Treatment also involves stopping or tapering steroid drops to reduce the immunocompromised state of the cornea and to engage the host's immune system. However, the protective nature of the crystalline deposits and the presence of biofilm can create a barrier that prevents antibiotics from penetrating the deeper layers of the cornea, leading to treatment resistance (Fulcher, 2001). In these cases, surgical excision with penetrating keratoplasty may be needed to clear the infection. Fortunately, a recent study showed significant success in managing ICK using topical linezolid 0.2%. The enhanced effectiveness of topical linezolid compared to vancomycin, which was previously used, might be due to its better tolerability and improved penetration of the biofilm (Tu, 2013).
References
Bowling, Brad. Kanski’s Clinical Ophthalmology: A Systemic Approach. Eighth Edition. Elsevier Edinburgh;2016.
Fulcher, T. P., Dart, J. K., McLaughlin-Borlace, L., Howes, R., Matheson, M., & Cree, I. (2001). Demonstration of biofilm in infectious crystalline keratopathy using ruthenium red and electron microscopy. Ophthalmology, 108(6), 1088–1092. https://doi-org.proxygw.wrlc.org/10.1016/s0161-6420(01)00561-9
Gorovoy, M. S., Stern, G. A., Hood, C. I., & Allen, C. (1983). Intrastromal noninflammatory bacterial colonization of a corneal graft. Archives of ophthalmology (Chicago, Ill. : 1960), 101(11), 1749–1752. https://doi-org.proxygw.wrlc.org/10.1001/archopht.1983.01040020751018
Kinota S, Wong KW, Biswas J, Rao NA. Changing patterns of infectious keratitis: overview of clinical and histopathologic features of keratitis due to acanthamoeba or atypical mycobacteria, and of infectious crystalline keratopathy. Indian J Ophthalmol. 1993; 41(1):3-14.
Porter, A. J., Lee, G. A., & Jun, A. S. (2018). Infectious crystalline keratopathy. Survey of ophthalmology, 63(4), 480–499. https://doi-org.proxygw.wrlc.org/10.1016/j.survophthal.2017.10.008
Tu, E. Y., & Jain, S. (2013). Topical linezolid 0.2% for the treatment of vancomycin-resistant or vancomycin-intolerant gram-positive bacterial keratitis. American journal of ophthalmology, 155(6), 1095–1098.e1. https://doi-org.proxygw.wrlc.org/10.1016/j.ajo.2013.01.010
Villani, E., Baudouin, C., Efron, N., Hamrah, P., Kojima, T., Patel, S. V., Pflugfelder, S. C., Zhivov, A., & Dogru, M. (2014). In vivo confocal microscopy of the ocular surface: from bench to bedside. Current eye research, 39(3), 213–231. https://doi-org.proxygw.wrlc.org/10.3109/02713683.2013.842592
Weisenthal RW, Afshari NA, Bouchard CS, Colby KA, Rootman DS, Tu EY, de Freitas D. BCSC External Disease and Cornea, Section 8. American Academy of Ophthalmology;2016.
Acknowledgments
Case Report Authors
Case 1: Siponimod-Associated Bilateral CME
Amanda Burns, MS3
Michael Frenkel, MS1
Case 2: Crystalline Keratopathy
Amir Elzomor, MS3
Thomas Hong, MS1
Resident Editors
Masumi Asahi, DO
Braedon Murdock, MD
Will Foos, MD
Attending Reviewer
David Belyea, MD MBA
Editors in Chief
Jason Dossantos, MS3
Julie Thomasian, MS4